Valid Names Results
Hypogeococcus pungens Granara de Willink, 1981 (Pseudococcidae: Hypogeococcus)Nomenclatural History
- Hypogeococcus festerianus; Williams 1973a: 565. misidentification (discovered by WilliaGr1992, 215).
- Hypogeococcus festerianus; McFadyen & Tomley 1978: 139. misidentification (discovered by WilliaGr1992, 215).
- Hypogeococcus festerianus; McFadyen 1979: 281. misidentification (discovered by WilliaGr1992, 215).
- Hypogeococcus pungens Granara de Willink 1981: 61. Type data: ARGENTINA: Province de Tucuman, Department of Trancas, Abril, on Alternanthera pungens. Holotype, female, Type depository: Tucuman: Fundacion e Instituto Miguel Lillo, Universidade Nactional de Tucuman, Argentina; accepted valid name Illustr.
- Hypogeococcus festerianus; Williams 1985a: 178. misidentification (discovered by WilliaGr1992, 215).
- Hypogeococcus festerianus; Suss & Trematerra 1986: 43. misidentification (discovered by RussoMa1997, 51).
- Hypogeococcus pungen Granara de Willink, 1981; Palma-Jiménez & Blanco-Meneses 2016: 127. misspelling of species epithet
Common Names
Ecological Associates
Hosts:
Families: 5 | Genera: 17
- Amaranthaceae
- Alternanthera | GermaiGr2005 MatileEt2006
- Alternanthera brasiliana | JoshiRaAm2024
- Alternanthera ficoidea | JoshiRaAm2024
- Alternanthera paronychioides | TriapiLoAg2014
- Alternanthera pungens | BenDov1994 Granar1981 TriapiLoAg2014 WilliaGr1992
- Gomphrena globosa | MatileEt2006 GermanKaSt2014
- Apocynaceae
- Mandevilla | DownieGu2004
- Cactaceae
- Acanthocereus tetragonus | BenDov1994 WilliaGr1992 | (=Acanthocereus pentagonus)
- Cactaceae | BenDovMaSt2002
- Cereus aethiops | BenDov1994 ClapsDe2001 WilliaGr1992
- Cereus forbesii | ClapsDe2001
- Cereus jamacaru | JoshiRaAm2024
- Cereus repandus | BenDovMaSt2002
- Cleistocactus | BenDov1994 ClapsDe2001 WilliaGr1992
- Consolea moniliformis | GermanKaSt2014
- Coryphantha elephantidens | JansenAl2023
- Espostoa | JansenAl2023
- Espostoa melanostele | JansenAl2023
- Harrisia bonplandii | JoshiRaAm2024 | (=Eriocereus bonplandii)
- Harrisia martinii | BenDov1994 WilliaGr1992 ClapsDe2001
- Harrisia pomanensis | ClapsDe2001
- Harrisia tortuosa | JoshiRaAm2024 | (=Eriocereus tortuosus)
- Lobivia | JansenAl2023
- Parodia microsperma | ClapsDe2001
- Selenicereus | JansenAl2023
- Soehrensia candicans | JansenAl2023 | (=Trichocereus candicans)
- Malvaceae
- Hibiscus rosa-sinensis | Malump2014a
- Portulacaceae
- Portulaca | BenDov1994 WilliaGr1992
- Portulaca oleracea | MatileEt2006
- Portulaca quadrifida | MatileEt2006
Foes:
Families: 2 | Genera: 3
- Coccinellidae
- Brumoides suturalis | JoshiRaAm2024
- Scymnus (Pullus) syoitii | JoshiRaAm2024
- Encyrtidae
- Anagyrus | TriapiLoAg2014
Geographic Distribution
Countries: 23
- Argentina
- Tucuman | BenDov1994 Granar1981
- Australia | PovedaAgLo2019
- Barbados | TriapiAgLo2018
- Brazil | BenDov1994 WilliaGr1992
- Canary Islands | JansenAl2023
- China | JansenAl2023
- Dominican Republic | GermanKaSt2014
- France | BenDovMaSt2002
- Greece | BenDovMaSt2002
- Guadeloupe | MatileEt2006
- Hawaiian Islands | GermanKaSt2014 | TriapiAgLo2018
- India
- Karnataka | ReddyMiMe2025
- Tamil Nadu | ReddyMiMe2025
- Telangana | ReddyMiMe2025
- Italy | LongoMaPe1995 MazzeoSuRu2008 RussoMa1997 SussTr1986
- Sicily | LongoRu1990
- Martinique | GermaiGr2005 MatileEt2006
- Monaco | BenDovMaSt2002
- Netherlands | JansenAl2023
- Paraguay | BenDov1994 WilliaGr1992
- Peru | BenDov1994 WilliaGr1992
- Puerto Rico & Vieques Island
- Puerto Rico | GermanKaSt2014
- Saint Lucia | Malump2014a
- Spain | BeltraSo2011
- U.S. Virgin Islands | TriapiAgLo2018
- United States
- California | TriapiAgLo2018
- Florida | DownieGu2004
Keys
- AhmedPoMi2025: pp.6 ( Adult (F) ) [Florida Pseudococcidae]
- Suh2019a: pp.2-6 ( Adult (F) ) [mealybugs interepted in S. Korea]
- WilliaGr1992: pp.203 ( Adult (F) ) [Central and South America]
- Granar1981: pp.64 ( Adult (F) ) [Americas]
Remarks
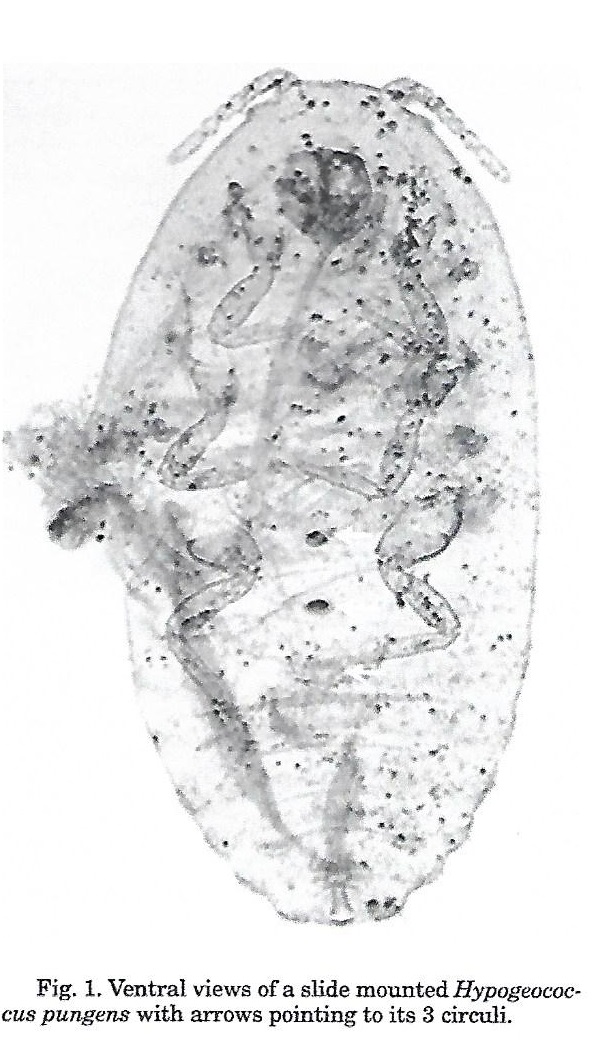
- Systematics: Hypogeococcus pungens differs from its congeners in having 3 circuli, whereas other species possess a single circulus (Williams & Granara de Willink, 1992).
Aguirre, et al. (2016) showed marked differences in biological parameters between populations of H. pungens collected on Amaranthaceae and the populations of H. pungens studied by McFadyen (1979) on Cactaceae. F1 of H. pungens sensu stricto (collected on Amaranthaceae in Argentina), even when they completed development to adulthood, did not produce viable second generation on Cactaceae under laboratory conditions. In addition to the differences in host range demonstrated in this study, “H. pungens” populations in Argentina and Puerto Rico also are different in the type of damage and the part of the plant attacked. None of the three cactus-attacking Hypogeococcus species in Argentina produce galls, whereas the mealybug pest of cacti in Puerto Rico produce galls. This suggests that the Hypogeococcus pest of cacti in Puerto Rico may be a different species than the “H. pungens” in Argentina. They propose that it is a new species that share the three circuli with H. pungens, and the specificity on cactus with H. festerianus.
The results of Proveda-Martinez (2019) indicated that mealybugs feeding on Amaranthaceae and Cactaceae collected in Argentina, on Cactaceae in Australia, and the Puerto Rico cactus pest, all referred to as H. pungens in the literature, are part of a species complex. Moreover, the genetic divergence in the H. pungens complex seemed to be strongly driven by the host plants. H. pungens sensu stricto appeared as a well-delimited species associated exclusively with the plants of the Amaranthaceae family in Argentina. However, the Puerto Rico cactus pest also appeared as part of the Amaranthaceae host clade, though genetically differentiated from all the populations from Argentina feeding on Amaranthaceae. The other members of the complex, which appeared to be ancestral of the mealybugs feeding on Amaranthaceae hosts, were specifically associated with Cactaceae. The study conducted by Poveda-Martinez, et al.(2020) revealed five major well supported clades. The first and second clades, both feeding on Amaranthaceae and/or Portulacaceae, appear closely related, the first encompassing H. pungens sensu stricto and the second mealybugs from northeastern Brazil, Puerto Rico, and United States (Florida). The third and fourth clades comprised cactus feeding mealybugs: the third from Argentina, Paraguay, and Australia and the fourth from southern Brazil and Puerto Rico. The fifth clade was an independent branch formed by Amaranthaceae-feeding mealybugs collected in southeastern Brazil.
Hypogeococcus pungens specimens from Australia are genetically similar to the Cactaceae clade of this species complex from Argentina. The insects collected in Australia can also reproduce parthenogenetically. Findings by Ezeh, et al. (2023) confirm that all populations of the mealy bug in Australia are not H. festerianus, but part of the H. pungens cryptic species complex.
- Structure: The morphology of all specimens in the H. pungens species complex in Australia that were examined by Ezeh, et al. (2023) were very similar. All specimens possessed three circuli, numerous multilocular disc pores throughout the entire body segments, and slender capitate setae on the head, thorax and anterior abdominal segments but lacked trilocular pores in all body segments. Genetic sequences for all specimens were identical and all phylogenies placed them within H. pungens rather than H. festerianus. Although the Australian population of Hypogeococcus should be regarded as H. pungens, it will likely require another name once the cryptic species complex is fully taxonomically revised.
- Biology: Occurring on roots and aerial parts of host plant (Suss & Trematerra, 1986). Reproductive compatibility experiments produced evidence supporting the hypothesis that H. pungens is a species complex and that Amaranthaceae and Cactaceae host clades are different species.(Poveda-Martinez, 2019)
- Economic Importance: Accounts by McFadyen & Tomley (1978), McFadyen (1979) and Williams (1985a) indicate that this species (under the name H. festerianus) is successful in Queensland, Australia, in controlling the Cactaceous plants Eriocereus bonplandii, E. martini, E. tortuosus and Acanthocereus pentagonus, since its introduction from Argentina as a biological control agent (Williams & Granara de Willink, 1992). In 1983, H. pungens was released in South Africa to control Cereus jamacaru de Candolle and H. martinii (Julien and Griffiths 1999, Zimmermann and Perez Sandi y Cuen 2010). In both countries, the control was successful, and since H. pungens was released, it has not been found in other family than Cactaceae. (Aguirre, et al., 2016) This species is causing severe damage in Puerto Rico to several pecies of native columnar cacti in the subfamily Cactoideae. Additional unpublished reports suggest that H. pungens is causing serious damage to native cactus plants in Puerto Rico. (German-Ramirez, et al., 2014) If the Harrisia cactus mealybug pest reported in Puerto Rico spreads beyond its current distribution range, species of cactus throughout the Caribbean region and North America, including Mexico, are at risk of being negatively affected by the insect. (Triapitsyn, et al., 2018)
- General Remarks: Good description and illustration of the adult female given by Granara de Willink (1981) and by Williams & Granara de Willink (1992). Good description and illustration of the larval instars given by Granara de Willink (1981). Genetic analyses indicate that the cactus feeding Hypogeococcus sp. mealybug introduced into Australia from Argentina was neither H. pungens sensu stricto nor H. festerianus. All species delimitation analyses suggested that mealybugs feeding on cacti, including the population exported to Australia, represent a new species. (Poveda-Martinez, 2019)
Illustrations
Citations
- AguirrDiCl2016: biological control, biology, description, distribution, economic importance, host, taxonomy, 1-7
- AguirrLoTr2019: host, taxonomy,
- AhmedPoMi2025: distribution, key, list of species, morphology, 3, 6
- BeltraSo2011: distribution, host,
- BeltraSoMa2012: molecular data, phylogenetics, 167-171
- BenDovMaSt2002: distribution, host, 186
- ClapsDe2001: distribution, host, 81
- DownieGu2004: distribution, host, molecular data, phylogeny, 258-259
- DuWaLi2025: evolution, genome, phylogenetics, taxonomy, 4
- EzehHeDa2023: genetics, taxonomy,
- EzehZaDa2025: biological control, ecology, host,
- FoldiGe2018: distribution, list, 12
- FrancoZaMe2009: economic importance,
- Gavril2018: reproduction, 222
- GermaiGr2005: distribution, host, 330
- GermanKaSt2014: distribution, economic importance, host, illustration, structure, taxonomy, 320-321
- Granar1981: description, distribution, host, illustration, taxonomy, 61-64
- HardyGuHo2008: molecular data, phylogeny, taxonomy, 51-71
- JansenAl2023: dispersal, host, 29
- JansenAl2023: dispersal, host, 35
- JoshiRaAm2024: DNA sequencing, biological control, diagnosis, dispersal, distribution, economic importance, host, illustration, record, 2
- KondoWa2022a: distribution, host, list, 23
- LiuNiLu2022: phylogenetics,
- LongoMaPe1995: distribution, 119
- Malump2014a: distribution, host, 80
- MatileEt2006: distribution, host, 178
- MazzeoLoPe2014: distribution, 58
- MazzeoSuRu2008: distribution, host, 149-152
- McFady1979: biological control, distribution, economic importance, host, life history, taxonomy, 281-287
- McFadyTo1981: biological control, distribution, economic importance, host, life history, taxonomy, 139-143
- Meurge2011: distribution, 81
- Millar2002: illustration, 207
- MuniapSuSe2026: biological control, 18
- PalmaJBl2016: morphology, phylogeny, 127, 131-132
- PellizChMi2015: distribution, 63,72
- PellizPo2014: distribution, 2
- PovedaAgLo2019: DNA sequencing, biology, control, distribution, genetics, phylogeny, taxonomy,
- PovedaAgLo2020: DNA, evolution, radiation, taxonomy,
- ReddyMiMe2025: dispersal, distribution, economic importance, host, illustration, 17
- RenAsHu2017: DNA, phylogeny, 4, 6
- RussoMa1997: distribution, 51
- SchutzGoGu2026: taxonomy, 6
- SongWaTa2024: phylogeny, 5, 8-9
- Suh2019a: key, 6
- SussTr1986: description, distribution, economic importance, host, taxonomy, 43-46
- TriapiAgLo2018: distribution, economic importance, host, 412
- TriapiLoAg2014: biological control, distribution, host, 201-231
- VonEll2025: distribution, 41
- WardOnBo2024: phylogenetics, 4
- Willia1973a: description, distribution, host, illustration, taxonomy, 565-569
- Willia1985a: catalog, description, distribution, host, illustration, taxonomy, 178
- WilliaGr1992: description, distribution, host, illustration, taxonomy, 212-215
- WilliaMi1999: life history, taxonomy, 524
- XuReLu2025: control, distribution, list, 66